Inflammation, Tissue Repair, and Cancer
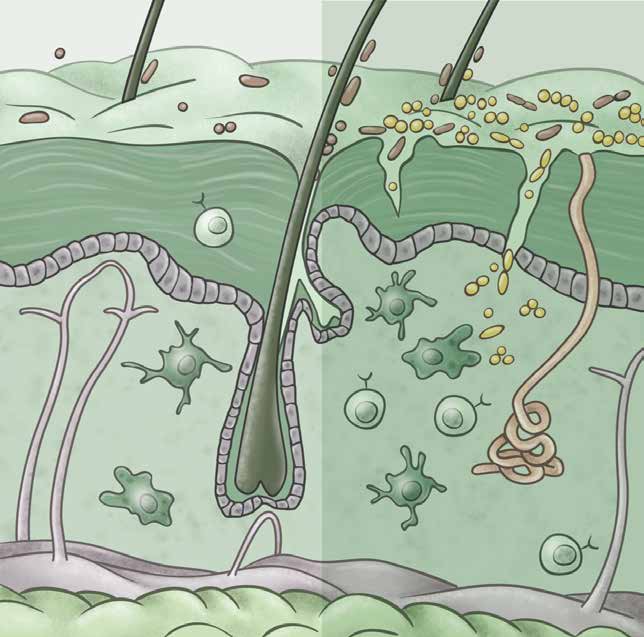
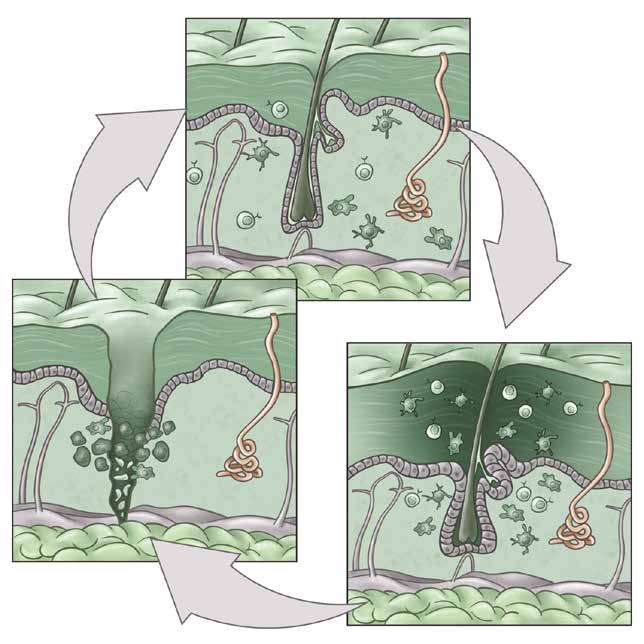
Our body’s epithelial barriers, such as the skin, lungs, and gut, interface with the external world and are routinely exposed to injurious, microbial, and carcinogenic stimuli. We study how barrier tissues sense, respond to, and remember these encounters. We have recently shown that the epithelia, and in particular epithelial stem cells, maintain a memory of inflammation. In this context, we examine how tissue adaptation promotes anti-pathogen responses and repair. On the other hand, maladaptations of inflammatory memory underlie recurrent autoimmune disease and cancers. We thus seek to understand the mechanisms controlling the delicate balance between beneficial and deleterious features of inflammatory memory.
We also aim to understand the role of immune cells in tissue repair and regeneration by studying mechanisms of immune-tissue crosstalk following acute damage and in chronic non-healing conditions such as skin ulcers and intestinal fistulae. Here, we take unbiased approaches to track the evolution of immune populations in wounds and then examine their pro- or anti-repair functions. Cancers have long been called “wounds that don’t heal” and so we parallel our studies in acute wounds with an understanding of cancer development and progression.