T-Cell Deletion of MyD88 Connects IL17 and IκBζ to RAS Oncogenesis
August 17, 2019
Christophe Cataisson, Rosalba Salcedo, Aleksandra M Michalowski, Mary Klosterman, Shruti Naik, Luowei Li, Michelle J Pan, Amalia Sweet, Jin-Qiu Chen, Laurie G Kostecka, Megan Karwan, Loretta Smith, Ren-Ming Dai, C Andrew Stewart, Lyudmila Lyakh, Wang-Ting Hsieh, Asra Khan, Howard Yang, Maxwell Lee, Giorgio Trinchieri, Stuart H Yuspa
Molecular Cancer Research
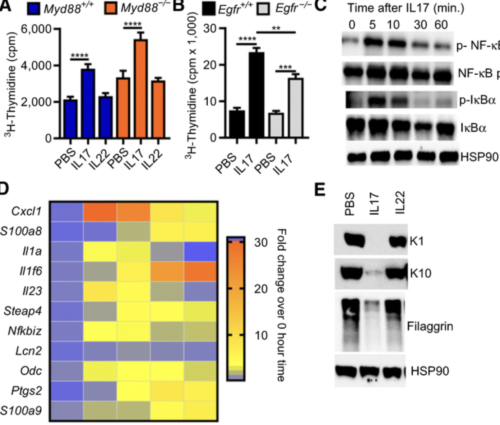
Keratinocytes or specific bone marrow subpopulations in oncogenic RAS-mediated skin carcinogenesis, we show that IL17 from infiltrating T cells and IκBζ signaling in keratinocytes are essential to produce a permissive microenvironment and tumor formation. Both normal and RAS-transformed keratinocytes respond to tumor promoters by activating canonical NF-κB and IκBζ signaling, releasing specific cytokines and chemokines that attract Th17 cells through MyD88-dependent signaling in T cells. The release of IL17 into the microenvironment elevates IκBζ in normal and RAS-transformed keratinocytes. Activation of IκBζ signaling is required for the expression of specific promoting factors induced by IL17 in normal keratinocytes and constitutively expressed in RAS-initiated keratinocytes. Deletion of Nfkbiz in keratinocytes impairs RAS-mediated benign tumor formation. Transcriptional profiling and gene set enrichment analysis of IκBζ-deficient RAS-initiated keratinocytes indicate that IκBζ signaling is common for RAS transformation of multiple epithelial cancers. Probing The Cancer Genome Atlas datasets using this transcriptional profile indicates that reduction of IκBζ signaling during cancer progression associates with poor prognosis in RAS-driven human cancers. IMPLICATIONS: The paradox that elevation of IκBζ and stimulation of IκBζ signaling through tumor extrinsic factors is required for RAS-mediated benign tumor formation while relative IκBζ expression is reduced in advanced cancers with poor prognosis implies that tumor cells switch from microenvironmental dependency early in carcinogenesis to cell-autonomous pathways during cancer progression.